ISMPP Job Board
The new and improved ISMPP Job Board provides members and their companies, professional employment recruiters, and non-members an exclusive opportunity to solicit new employees in a target-rich environment and provide leads for those currently in the job market.
NEW! Your job posting posts to ISMPP's LinkedIn and Facebook pages. And you have the option to distribute your posting via email to the ISMPP database.
Submit a Job Posting
View the New Job Board
Fee Structure and Ad Details*
ISMPP has the following fee structure based on posting duration and be sure to check out the UPGRADES!
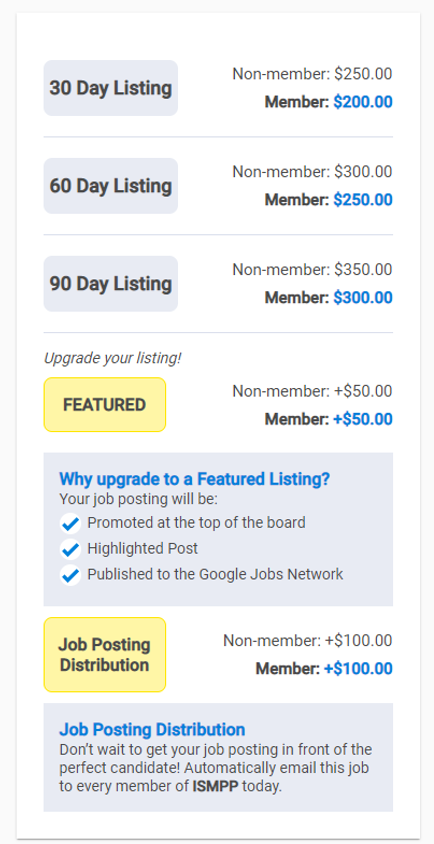
PLEASE NOTE: If you are an ISMPP member OR you are submitting an ad on behalf of a member/member company, you will need their log in information to access the Members Lounge. This will ensure you are charged at the 'Member Rate'.
Back to top
|